
Brand Name : Xultophy 100/3.6 pen
Marketing Authorization Holder : Novo Nordisk
℞ Prescription Required
Temp Controlled Express Courier
Prescription Xultophy 100/3.6 is an injectable medication for people with type 2 diabetes to lower blood sugar. Xultophy is recommended along with a good diet and exercise regime.
Xultophy pens contains 2 proven medications in 1 injector. It provides 24 hour blood sugar control and lowers A1C significantly.
Xultophy pens are not the first line of treatment for diabetes. It is never used in type 1 diabetes or in children.
Follow carefully the instructions in the packaging and take exactly as required by your doctor. Do not adjust the Xultophy dose without your doctors instruction. Monitor and keep track of your blood sugar carefully and share your results with your doctor.
Inject Xultophy at the same time daily without regards to food. Dosing may change according your Xultophy results as your requirement may change depending upon stress, illness, weight changes, or any lifestyle, diet and exercise changes you make in your life. Other medicines may also alter your required dose of Xultophy. The maximum allowable dose is 50 units per day. Do not inject into a vein or muscle. Do not use with an insulin pump.
Do not share your Xultophy and do not take if you have low blood sugar.
In 2019, when comparing Xultophy pen price to USA Xultophy coupon price, InsulinHub was found to offer a discount of approximately 50% in Xultophy savings.
Buy Xultophy 100/3.6 pens from InsulinHub.com for the best Xultophy price.
This product requires special packaging to maintain its integrity during the shipping process. DO NOT USE THIS MEDICATION if the attached temperature indicator shows that the medication was exposed to temperatures below 2 degrees or above 8 degrees Celsius, and contact the pharmacy immediately.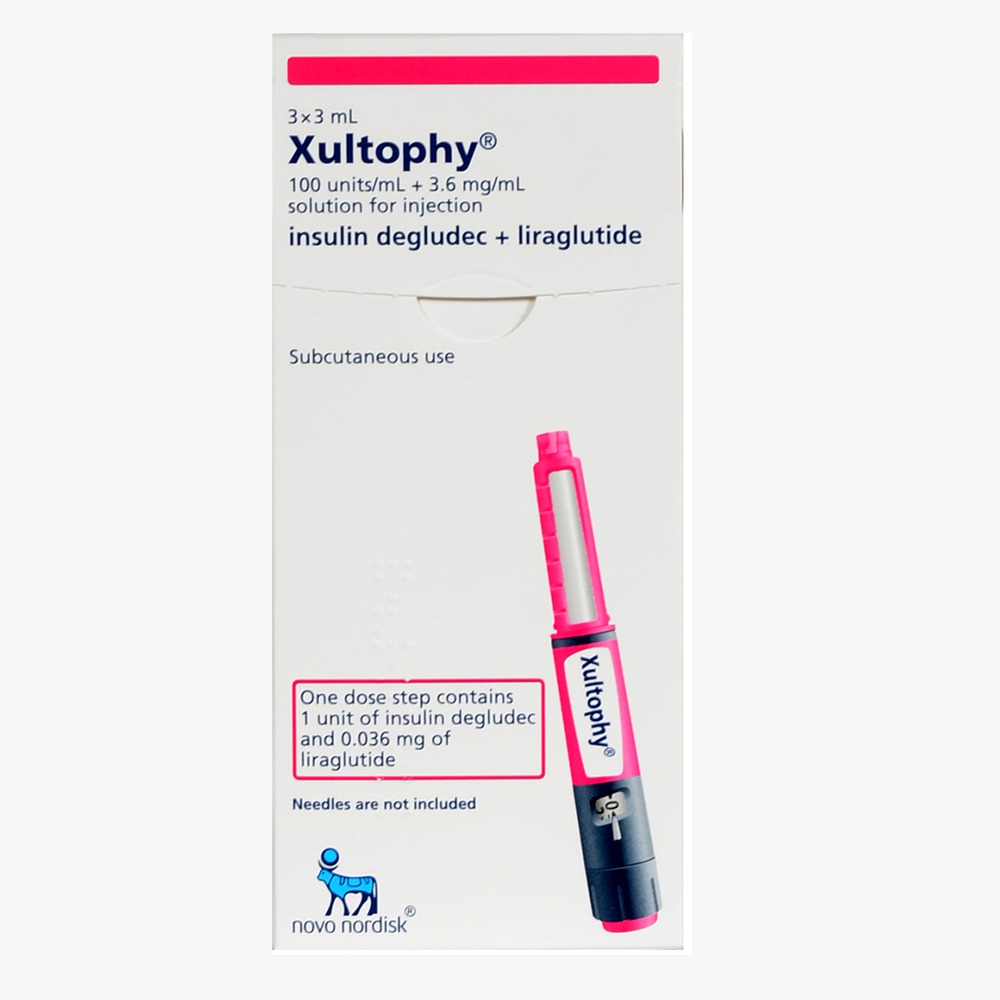
The product may appear different from
the product illustrated here

|

|

|

|